When we discuss the risk of telomere length to cancer risk, there are many studies but the conclusions we reach are limited by our simplistic assumptions.
On the one hand, the 2010 JAMA prospective study of 787 people showed shorter telomeres were associated with earlier and more severe telomere shortening.
On the other hand, a study published in the American Journal of Epidemiology of 28,000 people in the Singaporean Chinese Health Study showed an increase in cancer incidence with both shorter and longer telomere lengths; a whopping 4,000 of them got cancer in the 22-year study period.
How can we reconcile these two studies?
Firstly, we assume that leukocyte telomere length (from blood cells) is a useful measure of cancer risk. This may be true only because they hold the same progenitors of mesenchymal stem cells (MSC). In other words, MSC’s make the hematopoetic ones so that the chromosome mutations and telomere inheritenance patterns would follow a shared ancestor as described in this modified “Redenbacher Effect” post that I wrote.
What can we say for certain?
- Telomeres shorten with each division.
- Each daughter cell inherits the genetic integrity and the telomere lengths (96 total) of its immediate mother.
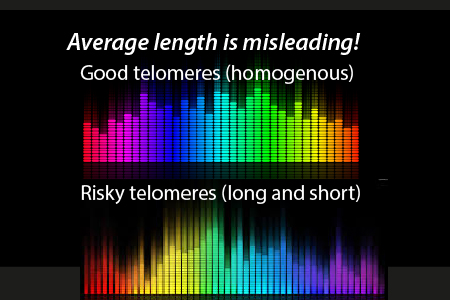
- Telomere shortening and relengthening. doesn’t happen equally in all 96 ends. That is why I liked the previous versions of the Life Length telomere tests that give us a visual representation of all of the telomere ends.
- The average length is misleading as it is more biologically significant to avoid extremely short ones that allow for mutation due to non-homologous end joining.
- Telomere lengthening continues even after critical shortening and mutation, leading to the U-shaped risk curve shown in the Chinese study.
- The preservation of the common progenitor integrity by maintenance, destruction, or preservation of stem cells what matters most.
- Mechanisms exist to trim excessively long telomeres as well.
What else can we safely assume?
- Cancer is more likely in older people because of accumulated mutations in stem cells as well as weakened immune surveillance.
- The ability of stem cells to undergo suicide (apoptosis) can only be impaired with age due to transciptional errors and chromosome-level mutations.
- The problem with stem cell transplantations such as those given after massive annihilation of bone marrow after leukeimia is that we didn’t preserve a perfect 25yo copy of the mesenchymal stem cells needed to give a “clean install” of the hematopoetic stem cells.
I hope the day is coming when adults can safely store their 25-yo copies and reliable thaw and replenish them. Right now, it’s like we are trying to destroy and maintain. But with the ability to restore good young copies, the problems of aging and bouncing back from serious disease will be more easily conquered.

