- Written:
- Author: Edward
- Posted in: dr ed park, osteoporosis, Podcasts, stem cell theory of aging
- Tags: bone health, Dr. Ed Park, helicase, osteoporosis, podcast, stem cell theory of aging, terc, wang
Like most conditions, poor telomerase activity occurs in cells which are under-performing.
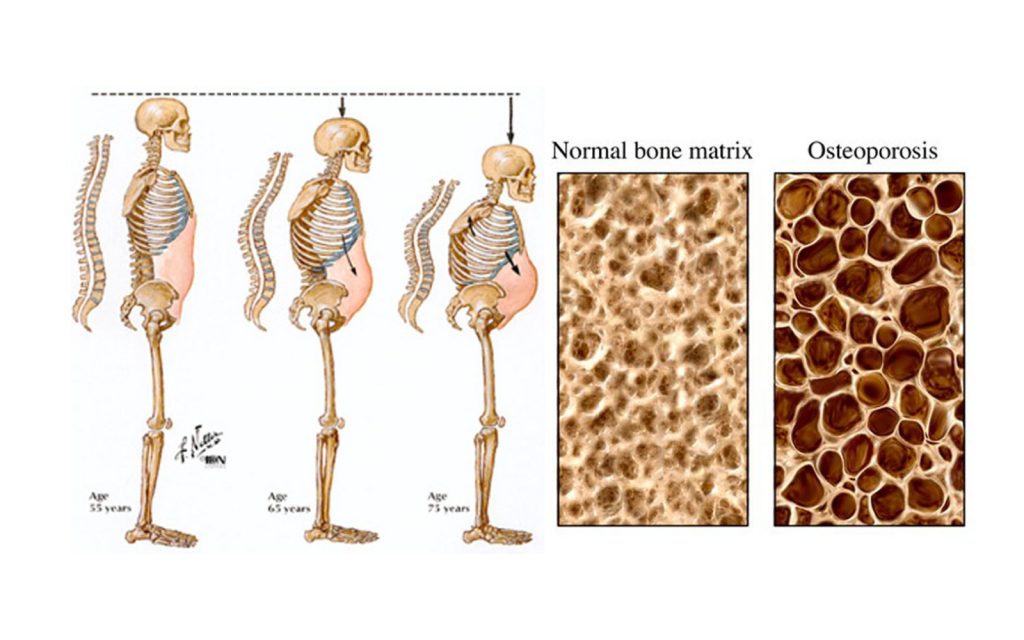
In this experiment, when the bone-making cells of mice were engineered to have a genetic deficiency in telomerase activity, the viability of those osteoblasts in terms of the ability to keep dividing suffered. They also knocked out a gene responsible for winding DNA and is defective a premature aging syndrome known as WRN RecQ Helicase.
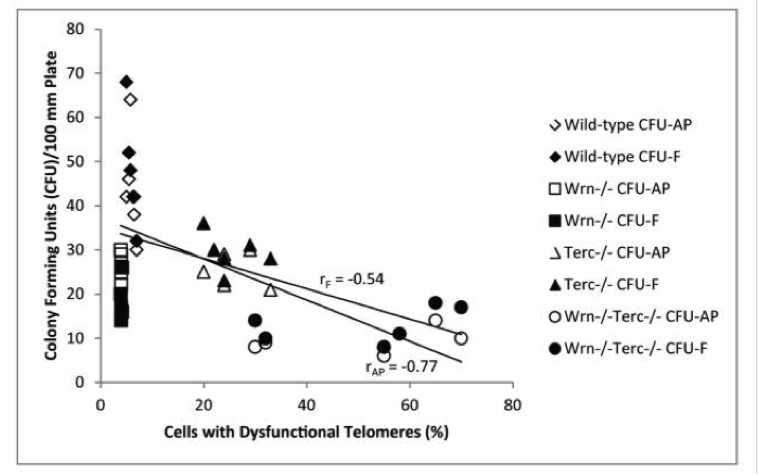
This diagram shows that the wild-type mice (diamonds) had low dysfunctional telomeres and high ability to reproduce (colony forming units). If you took away the helicase (squares), they had low ability to reproduce with low levels of dysfunctional telomeres (which shorten only when cell division is allowed to occur).
If you knocked out just the TERC, the key required for telomerase to work (triangles) you got high telomere dysfunction (short and damaged) and low ability to reproduce.
And if you knocked out both helicase and TERC (circles) you got the lowest ability to reproduce and very high rates of telomere dysfunction.
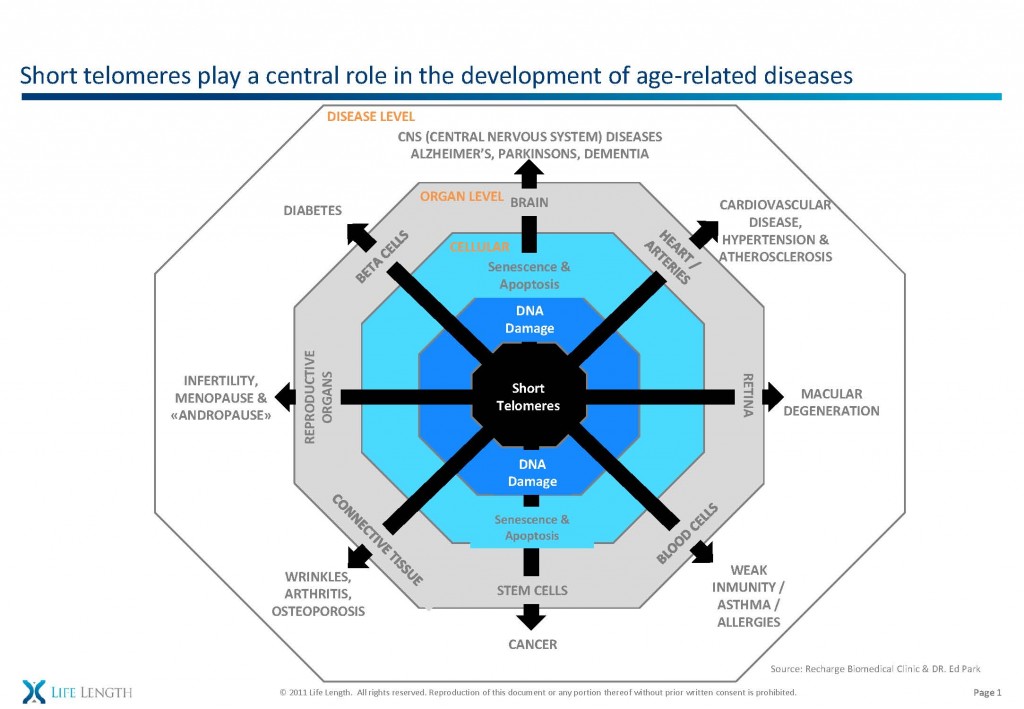
This is in keeping with my stem cell theory of aging in the bone – replicative senescence and the resultant DNA damage results in less viable bone-producing cells and osteoporosis. But the same process is at work in all organs.
For more information, watch this webinar I did on osteoporosis:

